ReActiv8
living
As for many patients, none of the treatment options for your Chronic Low Back Pain (CLBP) have brought you lasting relief. ReActiv8® is a different type of restorative therapy that addresses the underlying multifidus muscle dysfunction that can lead to mechanical CLBP. If you suffer from mechanical CLBP, ReActiv8 is designed to provide you with meaningful, long-term relief.
No one should live their lives in constant pain. If you have mechanical CLBP, it’s time to treat the cause of your pain, not only the symptoms. ReActiv8 Restorative Neurostimulation therapy activates the stabilizing muscles of your low back, focusing on the cause of your pain.


ReActiv8
A restorative treatment with transformative results
For many patients, ReActiv8 goes straight to the source of mechanical CLBP: your multifidus muscle, the primary stabilizer of your lumbar spine. If you suffer from mechanical CLBP, you may be all too familiar with the cycle of pain, muscle deterioration, and movement difficulty. ReActiv8’s restorative therapy can disrupt this cycle of pain and muscle degeneration by painlessly stimulating the nerves of your multifidus muscle and causing beneficial contractions. The ReAtiv8-B clinical study shows that this can significantly reduce mechanical CLBP.
Is ReActiv8 right for you?
Conditions for participating in the treatment.
1. ReActiv8 placement
During a minimally-invasive procedure, your physician will implant the ReActiv8 system in your lower back. Two electrical leads are positioned to stimulate the nerves that control your multifidus muscle, a stabilizing muscle in your lower back. The leads are connected to the implantable pulse generator which is placed just under the skin in the upper buttock or lower back area.
2. Patient-controlled therapy
Twice a day, you simply use a remote linked to your ReActiv8 system to start your therapy session — then lie down and relax. For each 30-minute session, ReActiv8 generates painless electrical pulses that cause your multifidus muscle to contract and relax.
3. Substantial and lasting improvements
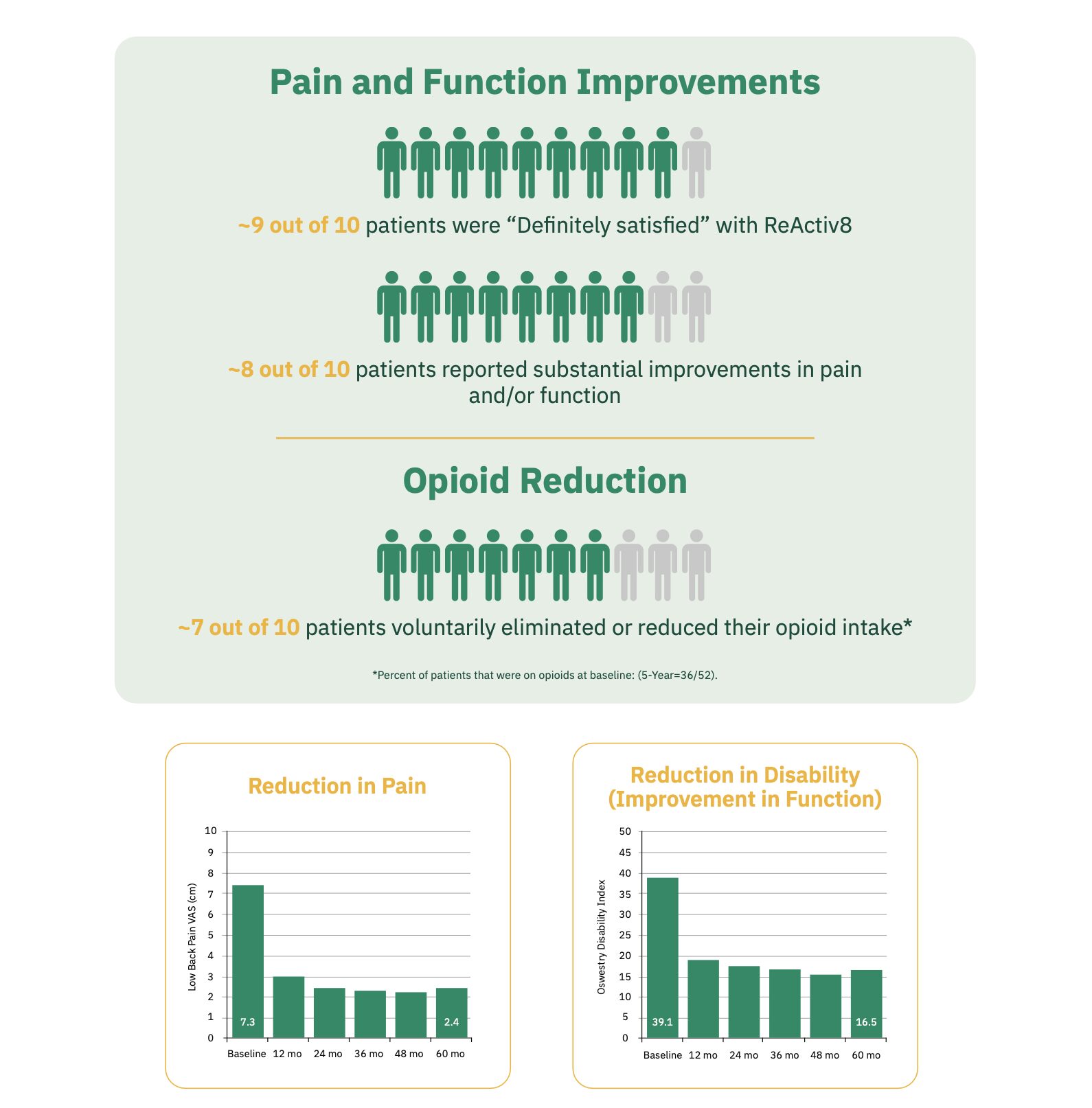
After one year, the ReActiv8-B clinical trial showed that 78% of patients were “definitely satisfied” with the treatment. Overall, 64% of patients had reduced their pain by half or more while 73% of participants had a 50% improvement in pain and/or disability. See the data from the clinical trial in the published manuscript in the PAIN Journal. [1]
1 – Gilligan C, Volschenk W, Russo M, et al. An implantable restorative-neurostimulator for refractory mechanical chronic low back pain: a randomized sham-controlled clinical trial. Pain 2021; Article in Press. doi: 10.1097/j.pain.0000000000002258
Backed by real patients with real results
Many people who were treated for their mechanical chronic low back pain with ReActiv8 have seen a dramatic improvement and were eager to share their stories to give hope to others. The following videos contain stories from three of our international patients who experienced remarkable results from the ReActiv8 system. Patients’ stories are theirs only and not indicative of the results for all patients. Patient outcomes will vary. See paragraph below for US indication and product information.
Frequently Asked Questions
Talk to your doctor to see if ReActiv8 is right for you.
ReActiv8 addresses the underlying multifidus muscle dysfunction that can lead to mechanical Chronic Low Back Pain (CLBP) by activating the nerves and muscle that control stability of the low back.
On average, people with mechanical CLBP who were treated with ReActiv8 had substantial improvements in pain, disability and quality of life after one year. See the long-term results in the PAIN Journal.
To assist in securing coverage from your health insurance provider, we offer the ReActiv8 Support and Verification Program (R.S.V.P.) by partnering with experts specialized in working with health insurance companies. To learn more about enrollment, talk to your doctor.
Phase 1: Discovery
Initial education on multifidus dysfunction, neuromuscular control, and ReActiv8
Mechanical chronic low back pain results from an injury or stress on the tissues surrounding the spine, including soft tissues, muscles, bones, and joints. Often times, this type of pain is due to impaired muscle control and neural inhibition of the multifidus, which is the largest stabilizing muscle in your back. When this neuromuscular inhibition occurs, there can be lack of spinal support, causing uncontrolled loading and pain.
Improving the neuromuscular control of the multifidus muscle can stabilize the spine, thereby increasing function and decreasing pain. ReActiv8 restorative neurostimulation is designed to address the underlying cause of mechanical low back pain by helping patients restore neuromuscular control of the multifidus muscle.
The multifidus muscle is the key stabilizing muscle in the lower back. Even a single injury to the back can cause impaired muscle control of the multifidus. This lack of muscle control can decrease the spine’s functional stability, leaving the spine susceptible to further injury and overloading. Restoring the neuromuscular control of the multifidus muscle can stabilize the spine, thereby increasing function and decreasing pain.
ReActiv8 is designed to address the underlying cause of mechanical low back pain by helping patients restore neuromuscular control of the multifidus muscle. The device is implanted underneath the skin during a minimally invasive outpatient procedure. A generator is connected to two electrical leads, which are positioned to stimulate the nerves that control your multifidus muscle. The patient controls the twice-daily therapy sessions, which can feel like a deep tissue massage.
- By stimulating the nerves that activate the multifidus muscle, ReActiv8 overcomes neural inhibition and allows the body to regain control of the multifidus, improving patient function and reducing mechanically based pain.
- The therapy program ReActiv8 uses is simple: using a remote control, the patient activates a 30-minute therapy session twice a day, which allows the system to contract and relax the multifidus muscle via the stimulation of the medial branch nerve. This presents a comfortable sensation that the patient can perform while resting in bed or on the couch.
- Once the patient obtains their goals of improved functionality and reduced pain, they can tailor the therapy usage to fit their needs. Some patients still use the therapy twice a day, while others use it as needed. A few even find that they can remove the system in a few years because they no longer require the therapy to keep them functional and pain free.
ReActiv8 is for patients who have mechanical chronic low back pain, but have not found relief through medical management or physical therapy. Candidates should not have had (and are not in need of) spine surgery. Your doctor can help determine if ReActiv8 is right for you.
Yes, ReActiv8 has been shown to provide significant and clinically meaningful benefits in disability, health-related quality of life, and pain in well-selected patients, irrespective of age.
ReActiv8 is for mechanical chronic low back pain and is not indicated for predominantly neuropathic pain such as radiculopathy or complex regional pain syndrome.
ReActiv8 is not indicated for patients that have previously had spine surgery in the lumbar region.
ReActiv8 is best suited for patients whose back pain is worse than their leg pain, assuming their leg pain does
not extend below the knee
Maintaining a healthy lifestyle is always important, however it is understandable that CLBP may prevent you from doing the amount of activity that you would like. Many patients in the ReActiv8 clinical trials were not able to exercise or successfully do physical therapy due to back pain, but with ReActiv8 therapy, were able to gain function so they could start getting back in shape.
The mechanism that causes radiating leg pain is different from back pain, and ReActiv8 is not indicated to treat the former (leg pain).
By enrolling in the RSVP program, Mainstay Medical can help increase your access to ReActiv8 through the submission of prior authorization and patient-based appeals facilitated by experts in this area.
Phase 2: Decision
Identified as a possible candidate & making the decision to get ReActiv8
Restorative treatments focus on improving function for patients by addressing the underlying cause. Palliative treatments focus on blocking pain through treatments like Spinal Cord Stimulation, oral medication, injections, or burning nerves (RFA, MBB).
ReActiv8 and SCS are different in almost every way. Most importantly, they each target different CLBP patient populations:
- ReActiv8 addresses musculoskeletal/mechanical/axial predominantly nociceptive CLBP.
- SCS addresses predominantly neuropathic CLBP and radiculopathy.
- They employ different mechanisms of action:
- ReActiv8 aims to restore multifidus motor control and functional segmental stability.
- SCS aims to interfere with the perception of pain with a palliative objective.
They have a completely different delivery schedule:
- ReActiv8 delivers stimulation to cause repetitive multifidus contractions during two 30-minute sessions daily.
- SCS typically delivers stimulation 24/7 to cover the pain.
There is no long-term data to support the use of SCS devices for mechanical CLBP. Physicians should be testing for multifidus dysfunction prior to any interventional therapy. If they test positive for multifidus dysfunction then restorative neurostimulation is the best treatment option. If there is no multifidus dysfunction, then an alternative therapy may make sense.
There is no long-term data to support the use of temporary PNS devices for mechanical CLBP. Physicians should be testing for multifidus dysfunction prior to any interventional therapy. If they test positive for multifidus dysfunction then restorative neurostimulation is the best treatment option. If there is no multifidus dysfunction, then an alternative therapy may make sense.
No. The ReActiv8 system is designed to stimulate the nerves that control the muscles that stabilize the lumbar spine. This results in a comfortable therapy that cycles deep muscular contractions, with the goal of restoring your neuromotor control of the lumbar spine. In contrast, TENS units attempt to block painful signals by transmitting electrical signals through your skin. This results in a completely different sensation and does not address the root cause of mechanical back pain.
ReActiv8 is designed to activate your deep stabilizing muscles correctly (physiologically). Radiofrequency ablation (RFA) destroys the nerves that connect the brain to the parts of the spine and deep muscles with the purpose of keeping painful signals from reaching the brain. Unfortunately, this also prevents the brain from communicating to these deep muscles to control spine stability.
ReActiv8 is the only neurostimulation therapy that works through a restorative mechanism, retraining your body to function naturally to support your spine. All other stimulators work by attempting to cover up the pain signals.
CT scans, fluoroscopy, ultrasound and X-Ray are all compatible with the ReActiv8 device. Special considerations must be made for MRI scans, however, and patients should review the ReActiv8 manual for more detailed guidance regarding MRIs.
ReActiv8 patients can receive an MRI. The ReActiv8 Implantable Pulse Generator (device) and 45 cm implantable leads (Model 8145) are MR Conditional devices with demonstrated safety in the MR environment only within specified conditions. Refer to the document ReActiv8 System Magnetic Resonance Imaging (MRI) Guidelines for an up-to-date list of approved MR Conditional components, model numbers, and required conditions. Failure to follow these guidelines for MRI scans may result in severe patient injury and/or device malfunction. Consult with your healthcare provider prior to an MR exam and inform MRI site personnel that you have an MR Conditional medical device during MR screening prior to the MR exam. Carry your device identification card with you at all times.
- Patients experience progressive long-term improvements in pain and function, over time, demonstrating a durable, consistent, and restorative result. This has been demonstrated through multiple clinical studies, with the largest randomized controlled trial demonstrating first of its kind results out to five years.
- ReActiv8 demonstrated favorable safety profiles compared to other neurostimulaton.
1. Gilligan et al. Five-Year Longitudinal Follow-up of Restorative Neurostimulation Demonstrates Durability of Effectiveness in Patients with Refractory Chronic Low Back Pain associated with Multifidus Muscle Dysfunction. https://doi.org/10.1016/j.neurom.2024.01.006
Phase 3: Preparing for Implant
Decision to receive ReActiv8 & preparation for procedure
While patients will typically be able to feel where the IPG is located with their hands, after 60-90 days, the body will heal around the IPG to the point where it is less perceptible. The patient may be able to see the location of the incisions, but won’t typically be able to see the IPG outline through their skin (when standing naturally) or over clothing. In comparison, neurostimulation systems such as pacemakers are typically much more noticeable due to where they are located.
ReActiv8 and SCS are different in almost every way. Most importantly, they each target different CLBP While everyone’s body is a little different, the nerve that is stimulated by the ReActiv8 leads is located in the same place in almost everyone. Your doctor will use x-ray images of your spine to obtain the best position of the leads to stimulate the nerve. The IPG (which also contains the battery pack) is generally implanted deep under the skin in the upper part of the buttock, below the belt line and above the seat line, or in the flank between the hips and the ribs, to make it as comfortable as possible. You should discuss the location of the IPG with your doctor before surgery.
Phase 4: Using the Therapy
Post-recovery process & how to use ReActiv8
After the implant procedure, you will have two incision sites, one central on the back about 1-2 inches in length and another at the location of the battery about 2 inches in length. The incisions will be closed using sutures with either glue, steri-strips, or a plastic incision closing device, then covered with a gauze dressing.
- Do not submerge in water until you have been cleared to by your doctor. You may shower 48 hours after your procedure, and the dressing should be changed with gauze and tape at that time. If the dressing gets saturated with blood within the first 24 hours, give your physician a call. Some bleeding on the dressing is expected. The dressing may be changed each day as needed after that first dressing change.
- For activity, we recommend to be out of bed as much as you can without exhausting yourself or worsening your pain.
- Walking is encouraged. Do not bend or twist to extremes. Do not lift anything heavier than a gallon of milk (about 10 pounds).
- Talk to your physician about pain control post-surgery. Many patients use Tylenol or NSAIDS along with ice for pain control, but your physician may have a specific plan for you.
- If using ice, ice for no more than 15 minutes at a time and wait for 15 minutes before reapplying ice to allow the skin to recover so it doesn’t get a freeze burn. DO NOT use heat until follow up.
- The steri-strips or glue will fall off on its own, so do not take them off yourself. Your doctor will remove anything needed at follow up and inspect the incision for proper healing.
Limit your activities to low or moderate levels during the first 4-6 weeks after implant. Light impact exercise such as walking or swimming is acceptable. Talk to your physician about what exercise may be right for you.
For the first 4-6 weeks, avoid lifting weights heavier than 10 pounds (e.g. a Gallon of milk).
Discuss with your physician ways to mitigate any pocket pain after the implant, as this should not persist for over a couple of weeks. Mild discomfort or awkward awareness of the pocket area typically subsides around 60-90 days.
Your wound will be checked around 10-14 days after surgery, where you will likely get your stitches removed. At this follow-up your system will be activated, and you will be instructed how to use your system and run therapy sessions. It is important to limit strenuous activity in the first 4-6 weeks to allow the system to heal.
You will require someone to take you home on the day of your implant, but you should be self-sufficient after the effects of anesthesia wear off.
The ReActiv8 device will not be turned on until the activation follow-up visit, about 10-14 days after surgery. At that time, the device stimulation will be programmed and individualized to patient therapy needs. The therapy starts with patients performing two, 30-minute sessions each day at convenient times. For most patients, this is typically once in the morning before getting out of bed and once in the evening when going to sleep or watching television.
Using a hand-held remote, the patient controls the twice-daily therapy sessions.
Most patients say the sessions feel like a pleasant series of deep muscle contractions, like flexing a muscle in an arm or leg, but in the lower back region. The system is programmed to be as comfortable as possible, so that it is easy and even enjoyable to perform the daily therapy sessions.
The therapy sessions are twice daily for 30 minutes each. The device has a timer on it and does not allow stimulation for more than 60 minutes per day.
The stomach is the most preferable position because it puts the least amount of pressure on the leads. However, the goal is for you to use the device as much as possible in the first year to achieve the best results. If the back works for you, talk to your Mainstay representative about programming the device in your preferred position to get the most out of your therapy.
It is preferred to perform sessions while laying down to allow your muscles to be fully relaxed. Some recliner chairs or other situations may be acceptable if your muscles can be fully relaxed for the duration of the therapy session.
Changing position may alter the distance between your leads and the stimulated nerves, causing a slight increase or decrease in stimulation and a different sensation. Talk to your therapy specialist about the best positions to perform your therapy.
It is important to perform as many of your prescribed sessions as you can in the first 6-9 months of therapy, however missing a session periodically will not alter your outcomes as long as you are using the majority of your sessions
The lockout period is designed to allow your muscles to rest and prevent overuse of stimulation. The system locks out for 2 hours after the first 30 min session, and for the rest of the day after the second session.
Yes, sometimes one side requires more energy output to correctly stimulate the nerve, and it takes longer to comfortably ramp this energy up to the programmed level. This can end up taking longer than the other side and therefore feel different.
Talk to your therapy specialist about the best position for stimulation. Performing sessions on your stomach or your back tend to give more even stimulation, but if you need to do sessions on your side your system can be programmed to give equal stimulation for a constantly used position.
The activator does not have the ability to pause a session. You can stop the session and start a new one at any time. The new session will run for a full 30 min unless you manually stop it.
Soft cloth lightly dampened with warm water.
The ReActiv8 system is tested to have enough battery life to last a minimum of 5 years with continuous use (two 30-minute session per day). However, in practice, ReActiv8 systems have shown to last much longer. If the battery ever does need replacement, a straightforward procedure to switch out the battery can be performed without having to replace the implanted leads.
No, the battery is a primary cell and does not need to be charged.
Phase 5: Long Term Expectations
Therapy compliance & setting expectations for programming, follow-ups, and outcomes.
The ReActiv8 follow-up schedule is tailored to customize the therapy to the patient’s body as it begins to restore control over the supporting muscles of the spine. It is an opportunity to gauge progress and celebrate the journey toward improved functionality and reduced pain. After the activation appointment, further follow- ups at 1, 3, and 6 months (or as needed) will be performed to tune your programming as the patient’s body 
- The majority of patients begin to feel improvement in their ability to function and perform activities with improved confidence between 6 and 16 weeks, but results can take longer than the average range in some patients. Patients report their back pain beginning to decrease shortly after they feel an improvement in function. The data from the clinical trials suggests that improvements seen in function and pain continue to accrue over time and are durable.
- To maximize the benefit of the therapy, patients are prescribed two therapy sessions a day for the first 12 months or until improvement is made. When significant improvement is made, most patients choose to tailor their therapy to fit their treatment needs and lifestyle.
- While some patients enjoy their therapy session every day in the long term, most tend to use their system when needed. A few patients have elected to have their system removed after a few years because they felt they had recovered.
- Physical Therapy prescription may also be encouraged in conjunction with ReActiv8.
Quality of life has been measured in multiple studies and shows dramatic improvements out to 5 years.
No. Patient reported outcomes from various real world studies out to 3 years are consistent with those found in the controlled clinical studies.
- The procedure is entirely reversible and the device can be removed by your physician.
- A small percentage of patients have elected to remove their device due to the resolution of symptoms after a few years of therapy.
Ask your physician first. It’s possible that chiropractic manipulation or deep tissue massage could damage your neurostimulator or dislodge the lead, which would cause you to need additional surgery to repair the system. It is generally advised to not attempt anything new/strenuous for the first 3 months as to not influence outcomes from Reactiv8.
ReActiv8 always allows for the possibility of any future surgery, as it does not alter the anatomy. However depending on the surgery you may be required to remove the ReActiv8 device.
Yes, you can walk through metal detectors or screening devices with an implanted neurostimulator. Do not linger inside, and try not to touch the sides of the screening device. Tell the security personnel that you have a “pacemaker for pain” and show your ReActiv8 Patient Identification Card. You may also request a hand search. If the search involves a hand-held security wand, ask them not to hold the wand near your neurostimulator any longer than needed.
Yes, assuming that your doctor has cleared your incision as fully healed.
The ReActiv8 system cannot be used with Diathermy. The ReActiv8 system has not been tested with other active devices and you should consult with your physician.
It is suggested to wait 4-6 weeks before any heavy lifting or strenuous activity to ensure proper healing of your implanted system. Afterwards, refrain from activities that may induce excessive stress on your leads or IPG. For example, jiu jitsu, rowing, etc.
The impact of pregnancy on ReActiv8 therapy has not been studied, although there was incidence of this during the ReActiv8-B study. We will turn the device off for the duration of your pregnancy and then we will turn it back on at a later time.
It is preferred to take your activator while traveling so you can continue your therapy. However, small interruptions in the therapy will not disrupt the results in the long term. Be aware that the system does not update time zones, so your 2 sessions within a 24- hour period may be shifted. Discuss with a Mainstay representative about how your sessions may be affected.
The device is not designed to go further than 5 meters (16 feet) in depth.
Yes, but make sure that the practitioner does not manipulate the area where the battery or leads are placed.
Due to the biocompatible materials used in the construction of ReActiv8, allergic reaction is unlikely. Your physician can assist with allergy testing if needed
While the device features protection from electromagnetic interference (EMI), some equipment can produce strong sources of EMI, e.g. transformers, electric welders etc., which may affect the device when in close proximity. Contact your physician if you have any questions.
Safety has not been established specifically with cryotherapy or infrared light therapy with vibration. Generally, these types of therapies are permitted, but note the following:
- Avoid manipulating or rubbing the implanted components of ReActiv8 through the skin. Manipulation may cause component damage, lead dislodgement, skin erosion, or unwanted stimulation at the implant site.
- Avoid using these treatments on an unhealed wound.
- While the device features protection from electromagnetic interference (EMI), some equipment can produce strong sources of EMI, e.g. transformers, electric welders, etc., which may affect the device when in close proximity. Contact your physician if you have any questions.
- Over 1000 patients have been implanted with ReActiv8 across AUS, US, UK, and Germany, including clinical trials and commercially. The development of ReActiv8 started in 2008, with clinical trials beginning in 2014. The long-term outcomes from the clinical trials are nearing the 10-year mark and many patients continue to see significant benefit from the therapy.
To learn more about the clinical studies surrounding the pathology and therapy, visit mainstaymedical.com/clinical.
Through the ReActiv8 Patient Ambassador Program, we offer the opportunity for patient-to-patient communications. You can sign up to talk to another patient by visiting patientpartner.com/mentor-match/reactiv8.
Restore You
Take the first step to see if ReActiv8 is right for you. Contact us to learn more.
Rev. # 06072024